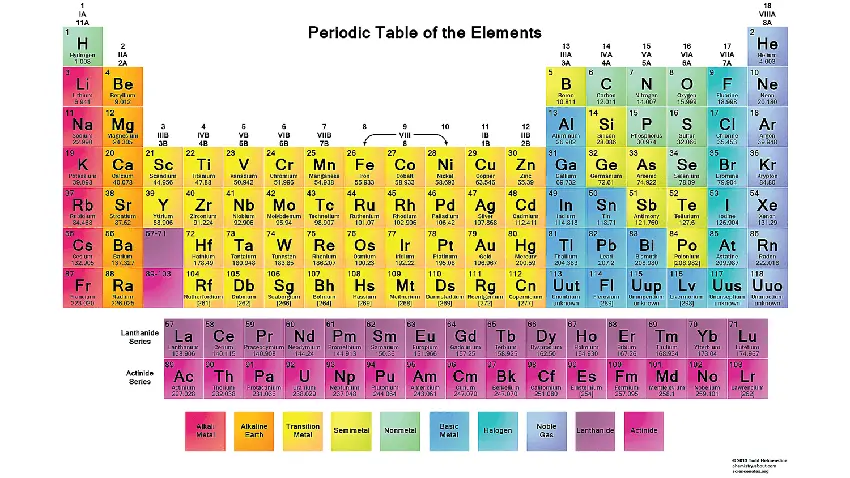
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is one of the most important tools in chemistry as it provides a systematic way to categorize and understand the behavior of elements. The periodic table allows scientists to predict the properties of elements, study their relationships, and identify trends in their chemical behavior.
Key features and components of the periodic table:
Elements: The periodic table lists all known chemical elements, which are substances composed of atoms with the same number of protons in their atomic nuclei. As of the latest knowledge, there are 118 known elements, ranging from hydrogen (the lightest) to oganesson (the heaviest).
Atomic Number: Each element in the periodic table is assigned a unique atomic number, represented by the symbol “Z.” The atomic number corresponds to the number of protons in the nucleus of an atom of that element.
Atomic Symbol: Each element is represented by a one- or two-letter chemical symbol. For example, “H” represents hydrogen, “C” represents carbon, and “O” represents oxygen.
Atomic Mass: The atomic mass of an element is the weighted average mass of its naturally occurring isotopes. It is usually listed beneath the element’s symbol in the periodic table.
Periods: The rows of the periodic table are called periods. Each period corresponds to the energy levels (shells) occupied by electrons in the atoms of elements in that row. The number of the period indicates the highest energy level that is partially filled with electrons.
You might like to know
Create an auto-grading quiz/assessment without any coding – try OnlineExamMaker today!
Groups: The columns of the periodic table are called groups or families. Elements within the same group share similar chemical properties because they have the same number of valence electrons (electrons in the outermost energy level).
Main Group Elements: Groups 1, 2, and 13 to 18 are known as the main group elements or representative elements. These elements have valence electrons in the s and p orbitals and exhibit a wide range of chemical behaviors.
Transition Metals: Groups 3 to 12 are known as transition metals. They have partially filled d orbitals and often exhibit multiple oxidation states.
Article outline
- Part 1: 30 periodic table quiz questions & answers
- Part 2: Download periodic table questions & answers for free
- Part 3: Free online quiz creator – OnlineExamMaker
Part 1: 30 periodic table quiz questions & answers
1. What is the organization of chemical elements based on their atomic number and recurring chemical properties called?
a) Atomic arrangement
b) Elemental order
c) Periodic table
d) Chemical matrix
Answer: c) Periodic table
2. How are elements arranged in the periodic table?
a) Alphabetical order
b) Increasing atomic mass
c) Decreasing atomic number
d) Increasing electronegativity
Answer: c) Decreasing atomic number
3. The atomic number of an element is determined by the number of:
a) Protons in its nucleus
b) Electrons in its nucleus
c) Neutrons in its nucleus
d) Nucleons in its nucleus
Answer: a) Protons in its nucleus
4. What is the total number of known elements in the periodic table?
a) 80
b) 92
c) 118
d) 150
Answer: c) 118
5. The rows of the periodic table are called:
a) Groups
b) Families
c) Periods
d) Series
Answer: c) Periods
6. What are the columns of the periodic table known as?
a) Series
b) Groups
c) Families
d) Blocks
Answer: b) Groups
7. Elements in the same group of the periodic table have the same number of:
a) Electrons in the outermost energy level
b) Protons in the nucleus
c) Neutrons in the nucleus
d) Valence electrons
Answer: d) Valence electrons
8. Which element has the atomic number 1 and is the lightest element in the periodic table?
a) Hydrogen
b) Helium
c) Carbon
d) Oxygen
Answer: a) Hydrogen
9. The chemical symbol “Na” represents which element in the periodic table?
a) Neon
b) Nickel
c) Nitrogen
d) Sodium
Answer: d) Sodium
10. Elements in Group 18 of the periodic table are known as:
a) Halogens
b) Noble gases
c) Alkaline earth metals
d) Transition metals
Answer: b) Noble gases
11. Which group of elements is known for their high reactivity and tendency to form salts?
a) Alkali metals (Group 1)
b) Alkaline earth metals (Group 2)
c) Halogens (Group 17)
d) Noble gases (Group 18)
Answer: c) Halogens (Group 17)
12. What is the name of the elements in Group 1 of the periodic table?
a) Alkali metals
b) Noble gases
c) Halogens
d) Alkaline earth metals
Answer: a) Alkali metals
13. Which group of elements is known for their high luster, malleability, and electrical conductivity?
a) Halogens (Group 17)
b) Transition metals (Groups 3-12)
c) Alkali metals (Group 1)
d) Metalloids (Along the staircase line)
Answer: b) Transition metals (Groups 3-12)
14. The elements in the two rows at the bottom of the periodic table are called:
a) Actinides and lanthanides
b) Noble gases and halogens
c) Alkali metals and alkaline earth metals
d) Metalloids and transition metals
Answer: a) Actinides and lanthanides
15. Elements in Group 2 of the periodic table are known as:
a) Halogens
b) Noble gases
c) Alkaline earth metals
d) Transition metals
Answer: c) Alkaline earth metals
Part 2: Download periodic table questions & answers for free
Download questions & answers for free
16. Which group of elements is known for their relatively low reactivity and full outer electron shells?
a) Alkali metals (Group 1)
b) Alkaline earth metals (Group 2)
c) Noble gases (Group 18)
d) Halogens (Group 17)
Answer: c) Noble gases (Group 18)
17. Which element has the chemical symbol “Fe” and is commonly used in making steel?
a) Iron
b) Iodine
c) Indium
d) Silicon
Answer: a) Iron
18. Which element is essential for life and forms the basis of organic molecules?
a) Iron
b) Oxygen
c) Carbon
d) Calcium
Answer: c) Carbon
19. The elements in the p-block of the periodic table include:
a) Groups 1 and 2
b) Groups 3 to 12
c) Groups 13 to 18
d) Groups 17 and 18
Answer: c) Groups 13 to 18
20. Elements in the same group of the periodic table have similar:
a) Atomic masses
b) Atomic numbers
c) Chemical properties
d) Numbers of protons
Answer: c) Chemical properties
21. Which group of elements is known for their ability to conduct electricity and heat well?
a) Halogens (Group 17)
b) Alkali metals (Group 1)
c) Transition metals (Groups 3-12)
d) Noble gases (Group 18)
Answer: c) Transition metals (Groups 3-12)
22. The element with the atomic number 6 is:
a) Nitrogen
b) Carbon
c) Oxygen
d) Helium
Answer: b) Carbon
23. Elements in Group 17 of the periodic table are known as:
a) Noble gases
b) Alkali metals
c) Halogens
d) Alkaline earth metals
Answer: c) Halogens
24. What element has the chemical symbol “He” and is a noble gas?
a) Helium
b) Hydrogen
c) Iron
d) Mercury
Answer: a) Helium
Just so you know
With OnlineExamMaker quiz software, anyone can create & share professional online assessments easily.
25. Which group of elements is known for their high reactivity and tendency to form strong bases when reacting with water?
a) Alkali metals (Group 1)
b) Alkaline earth metals (Group 2)
c) Halogens (Group 17)
d) Noble gases (Group 18)
Answer: b) Alkaline earth metals (Group 2)
26. What is the name of the elements in Group 18 of the periodic table?
a) Halogens
b) Noble gases
c) Alkali metals
d) Alkaline earth metals
Answer: b) Noble gases
27. Which element has the atomic number 17 and is a member of the halogen group?
a) Chlorine
b) Carbon
c) Calcium
d) Copper
Answer: a) Chlorine
28. The elements in the d-block of the periodic table include:
a) Alkali metals (Group 1)
b) Noble gases (Group 18)
c) Halogens (Group 17)
d) Transition metals (Groups 3-12)
Answer: d) Transition metals (Groups 3-12)
29. What is the name of the elements in Group 1 of the periodic table?
a) Noble gases
b) Alkali metals
c) Alkaline earth metals
d) Transition metals
Answer: b) Alkali metals
30. What is the term for elements that are located along the “staircase” line in the periodic table and have properties intermediate between metals and nonmetals?
a) Noble gases
b) Halogens
c) Metalloids
d) Transition metals
Answer: c) Metalloids
Part 3: Best online quiz making platform – OnlineExamMaker
OnlineExamMaker is cloud-baed and mobile friendly, the created exams can be access on various devices, including desktop computers, laptops, smartphones, and tablets, allowing you to test your connection speed anytime, anywhere. You can personalize your quizzes by adding your branding elements such as logos, colors, and custom backgrounds. You can also set time limits, randomize question order, and customize feedback messages based on learners’ responses.
Create Your Next Quiz/Exam with OnlineExamMaker