The ozone layer is a protective shield in Earth’s stratosphere, composed of ozone (O3) molecules that absorb most of the sun’s harmful ultraviolet (UV) radiation. This thin layer, located roughly 10 to 50 kilometers above the surface, plays a vital role in safeguarding life by preventing UV rays from reaching the ground, which could otherwise cause skin cancer, cataracts, and damage to ecosystems. Formed naturally through the interaction of sunlight with oxygen molecules, the ozone layer has been significantly depleted in certain areas due to human-made chemicals like chlorofluorocarbons (CFCs). International efforts, such as the Montreal Protocol, have helped reduce these emissions, allowing gradual recovery, but challenges like climate change continue to threaten its stability.
Table of contents
- Part 1: OnlineExamMaker AI quiz generator – The easiest way to make quizzes online
- Part 2: 20 ozone layer quiz questions & answers
- Part 3: Try OnlineExamMaker AI Question Generator to create quiz questions
Part 1: OnlineExamMaker AI quiz generator – The easiest way to make quizzes online
Are you looking for an online assessment to test the ozone layer knowledge of your learners? OnlineExamMaker uses artificial intelligence to help quiz organizers to create, manage, and analyze exams or tests automatically. Apart from AI features, OnlineExamMaker advanced security features such as full-screen lockdown browser, online webcam proctoring, and face ID recognition.
Take a product tour of OnlineExamMaker:
● Includes a safe exam browser (lockdown mode), webcam and screen recording, live monitoring, and chat oversight to prevent cheating.
● AI Exam Grader for efficiently grading quizzes and assignments, offering inline comments, automatic scoring, and “fudge points” for manual adjustments.
● Embed quizzes on websites, blogs, or share via email, social media (Facebook, Twitter), or direct links.
● Handles large-scale testing (thousands of exams/semester) without internet dependency, backed by cloud infrastructure.
Automatically generate questions using AI
Part 2: 20 ozone layer quiz questions & answers
or
1. Question: What is the ozone layer?
Options:
A) A layer of oxygen in the atmosphere that absorbs ultraviolet radiation
B) A layer of carbon dioxide that traps heat
C) A layer of nitrogen that supports plant life
D) A layer of water vapor that causes rain
Answer: A
Explanation: The ozone layer is a region in the Earth’s stratosphere composed of ozone (O3) molecules, which absorb most of the Sun’s harmful ultraviolet (UV) radiation, protecting life on Earth.
2. Question: Where is the ozone layer primarily located?
Options:
A) Troposphere
B) Stratosphere
C) Mesosphere
D) Thermosphere
Answer: B
Explanation: The ozone layer is mainly found in the stratosphere, approximately 10 to 50 kilometers above the Earth’s surface, where it plays a crucial role in filtering UV radiation.
3. Question: What is the primary function of the ozone layer?
Options:
A) To absorb ultraviolet radiation from the Sun
B) To regulate global temperatures
C) To provide oxygen for breathing
D) To form clouds and precipitation
Answer: A
Explanation: The ozone layer protects living organisms by absorbing the majority of the Sun’s harmful ultraviolet radiation, reducing the risk of skin cancer, cataracts, and other health issues.
4. Question: What is the main cause of ozone layer depletion?
Options:
A) Chlorofluorocarbons (CFCs) released from aerosols and refrigerants
B) Natural volcanic eruptions
C) Increased carbon dioxide levels
D) Deforestation
Answer: A
Explanation: CFCs and other ozone-depleting substances break down ozone molecules in the stratosphere when exposed to UV radiation, leading to thinning of the ozone layer.
5. Question: What are CFCs?
Options:
A) Chemicals used in refrigeration and aerosols that deplete ozone
B) Natural gases produced by oceans
C) Pollutants from car exhaust
D) Elements in the Earth’s core
Answer: A
Explanation: Chlorofluorocarbons (CFCs) are synthetic compounds once widely used in products like air conditioners and spray cans; they rise to the stratosphere and release chlorine atoms that destroy ozone molecules.
6. Question: When was the Montreal Protocol adopted?
Options:
A) 1987
B) 1970
C) 1997
D) 2005
Answer: A
Explanation: The Montreal Protocol, adopted in 1987, is an international treaty designed to phase out the production and consumption of ozone-depleting substances like CFCs.
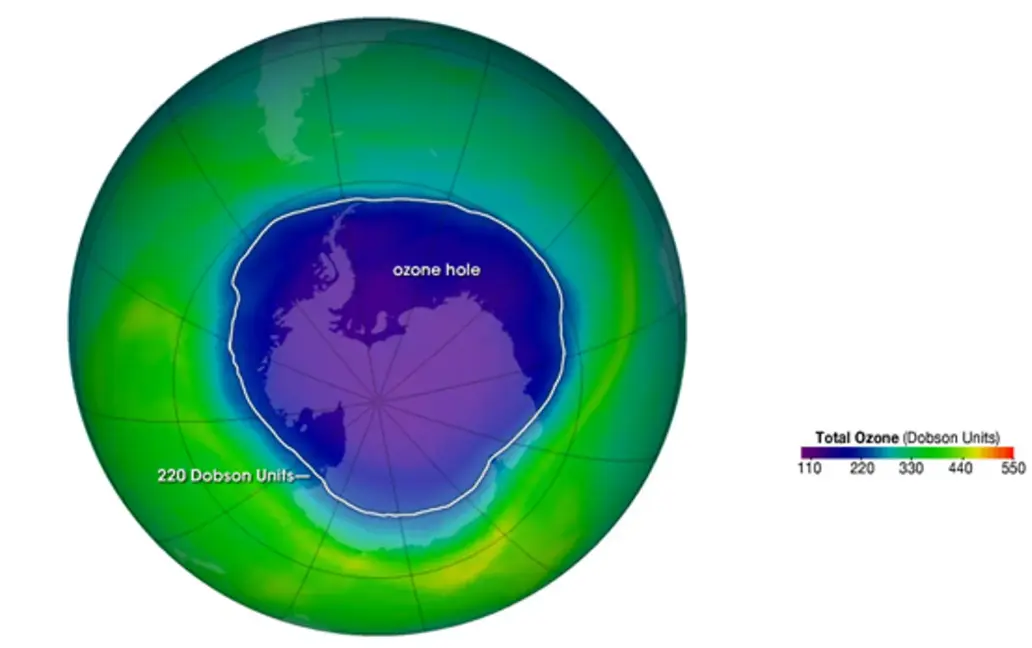
7. Question: What is the ozone hole?
Options:
A) A region of severe ozone depletion, mainly over Antarctica
B) A natural gap in the atmosphere
C) An increase in ozone concentration
D) A layer of polluted air
Answer: A
Explanation: The ozone hole refers to a significant thinning of the ozone layer, particularly over Antarctica during spring, caused by chemical reactions involving CFCs and polar stratospheric clouds.
8. Question: How does ozone depletion affect human health?
Options:
A) Increases risk of skin cancer and cataracts due to more UV exposure
B) Causes respiratory diseases directly
C) Leads to vitamin deficiencies
D) Reduces life expectancy through climate change
Answer: A
Explanation: Ozone depletion allows more ultraviolet radiation to reach the Earth’s surface, increasing the incidence of skin cancer, eye damage like cataracts, and weakening the immune system.
9. Question: What role do CFCs play in ozone depletion?
Options:
A) They release chlorine atoms that catalytically destroy ozone molecules
B) They directly replace ozone in the atmosphere
C) They block UV radiation
D) They enhance ozone formation
Answer: A
Explanation: CFCs break down in the stratosphere under UV light, releasing chlorine atoms that act as catalysts, breaking apart thousands of ozone molecules without being consumed.
10. Question: Which international agreement aims to protect the ozone layer?
Options:
A) Montreal Protocol
B) Kyoto Protocol
C) Paris Agreement
D) Basel Convention
Answer: A
Explanation: The Montreal Protocol is a global treaty that successfully regulates the production and use of substances that deplete the ozone layer, leading to its gradual recovery.
11. Question: How is ozone formed in the stratosphere?
Options:
A) Through the reaction of oxygen molecules with UV radiation
B) From carbon dioxide breakdown
C) By volcanic activity
D) From industrial emissions
Answer: A
Explanation: Ozone is formed when ultraviolet radiation from the Sun splits oxygen molecules (O2) into atoms, which then combine with other O2 molecules to create O3 (ozone).
12. Question: What is the primary effect of ozone depletion on marine life?
Options:
A) It harms phytoplankton, which forms the base of the ocean food chain
B) It increases ocean acidity
C) It causes more frequent tsunamis
D) It reduces water temperature
Answer: A
Explanation: Increased UV radiation from ozone depletion can damage phytoplankton, affecting the entire marine ecosystem and potentially disrupting fish populations and global food chains.
13. Question: Why is the ozone layer thinner over Antarctica?
Options:
A) Due to cold temperatures and polar stratospheric clouds that enhance chemical reactions
B) Because of higher pollution levels in the region
C) From natural solar variations
D) Due to excessive human activity
Answer: A
Explanation: The extreme cold in Antarctica forms polar stratospheric clouds that provide surfaces for reactions between CFCs and ozone, accelerating depletion and creating the ozone hole.
14. Question: What has been the trend in ozone layer recovery since the 1990s?
Options:
A) Gradual recovery due to reduced CFC use
B) Continued rapid depletion
C) No significant change
D) Complete restoration
Answer: A
Explanation: International efforts like the Montreal Protocol have led to a decline in CFC production, allowing the ozone layer to slowly recover, with projections for full recovery by mid-century.
15. Question: How does the ozone layer relate to climate change?
Options:
A) Ozone depletion can exacerbate global warming by allowing more UV radiation
B) It directly causes greenhouse gas emissions
C) It cools the planet by reflecting sunlight
D) It has no connection
Answer: A
Explanation: While the ozone layer primarily protects against UV radiation, its depletion can indirectly influence climate patterns by altering atmospheric temperatures and circulation.
16. Question: What is the Dobson unit used to measure?
Options:
A) The thickness of the ozone layer
B) The level of CFCs in the atmosphere
C) Global temperature changes
D) UV radiation intensity
Answer: A
Explanation: The Dobson unit measures the total ozone in a column of air from the Earth’s surface to the top of the atmosphere, indicating the thickness of the ozone layer.
17. Question: Who discovered the Antarctic ozone hole?
Options:
A) British Antarctic Survey scientists in 1985
B) NASA researchers in 1970
C) European Space Agency in 2000
D) United Nations in 1990
Answer: A
Explanation: In 1985, scientists from the British Antarctic Survey detected the first significant ozone hole over Antarctica using ground-based measurements.
18. Question: What are alternatives to ozone-depleting substances like CFCs?
Options:
A) Hydrofluorocarbons (HFCs) and other non-ozone-depleting chemicals
B) More CFCs with modifications
C) Natural gases from plants
D) Increased use of aerosols
Answer: A
Explanation: HFCs and other substitutes have been developed as alternatives to CFCs for use in refrigeration and aerosols, though they may have their own environmental impacts like contributing to global warming.
19. Question: How does UV radiation affect ecosystems due to ozone depletion?
Options:
A) It damages DNA in plants and animals, reducing biodiversity
B) It increases plant growth
C) It has no effect on ecosystems
D) It only affects urban areas
Answer: A
Explanation: Increased UV radiation from ozone depletion can cause mutations in DNA, leading to reduced growth in plants, lower crop yields, and harm to aquatic life, thus threatening biodiversity.
20. Question: What is the predicted future for the ozone layer?
Options:
A) Full recovery by the latter half of the 21st century if current protocols are followed
B) Permanent depletion
C) Rapid expansion
D) No prediction possible
Answer: A
Explanation: Scientific models predict that with continued adherence to the Montreal Protocol, the ozone layer will return to 1980 levels by around 2060-2070, though challenges like climate change may influence this.
or
Part 3: Try OnlineExamMaker AI Question Generator to create quiz questions
Automatically generate questions using AI