Electrochemistry is a branch of chemistry that deals with the study of the relationship between electrical energy and chemical reactions. It focuses on the processes in which electrical energy is converted into chemical energy and vice versa. These electrochemical processes occur at the interface between an electronic conductor (usually a metal or a semiconductor) and an ionic conductor (typically an electrolyte solution).
Key Concepts in Electrochemistry:
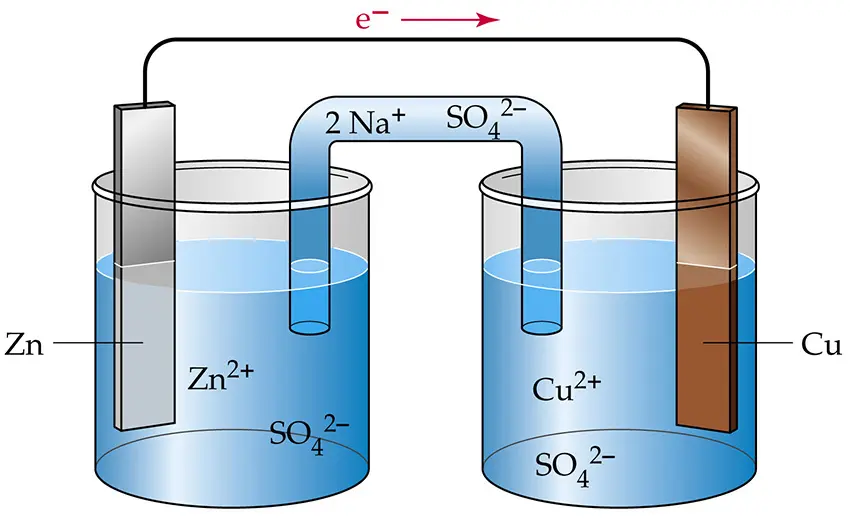
Electrochemical Cells: An electrochemical cell is a device that converts chemical energy into electrical energy or vice versa. There are two main types of electrochemical cells: galvanic (voltaic) cells and electrolytic cells.
Redox Reactions: Electrochemistry is closely linked to redox (reduction-oxidation) reactions, where one species undergoes reduction (gain of electrons) and another undergoes oxidation (loss of electrons).
Electrode: An electrode is a conductor through which electricity enters or leaves an electrochemical cell. There are two types of electrodes: the anode (where oxidation occurs) and the cathode (where reduction occurs).
Half-Cell: A half-cell is one of the two compartments in an electrochemical cell, consisting of an electrode immersed in an electrolyte solution.
Electrolyte: An electrolyte is a solution that contains ions and can conduct electricity.
Pro Tip
You can build engaging online quizzes with our free online quiz maker.
Table of content
- Part 1: 30 Electrochemistry quiz questions & answers
- Part 2: Download Electrochemistry questions & answers for free
- Part 3: Free online quiz platform – OnlineExamMaker
Part 1: 30 Electrochemistry quiz questions & answers
1. What is electrochemistry?
a) The study of electricity in gases
b) The study of electrical engineering principles
c) The study of the relationship between electrical energy and chemical reactions
d) The study of nuclear reactions
Answer: c) The study of the relationship between electrical energy and chemical reactions
2. What is an electrochemical cell?
a) A device that converts electrical energy into mechanical energy
b) A device that converts electrical energy into chemical energy
c) A device that converts chemical energy into mechanical energy
d) A device that converts chemical energy into electrical energy
Answer: d) A device that converts chemical energy into electrical energy
3. In an electrochemical cell, where does oxidation occur?
a) Anode
b) Cathode
c) Electrolyte
d) Electromotive force
Answer: a) Anode
4. What is the half-cell in an electrochemical cell?
a) The compartment where oxidation occurs
b) The electrode where reduction occurs
c) One of the two compartments in an electrochemical cell
d) The compartment where electrolysis takes place
Answer: c) One of the two compartments in an electrochemical cell
5. Which type of cell produces electrical energy through spontaneous redox reactions?
a) Electrolytic cell
b) Galvanic cell (Voltaic cell)
c) Fuel cell
d) Solar cell
Answer: b) Galvanic cell (Voltaic cell)
6. What is the process called where a metal is coated with a layer of another metal using electricity?
a) Oxidation
b) Reduction
c) Electroplating
d) Electrolysis
Answer: c) Electroplating
7. What is the standard electrode potential?
a) The potential difference between two electrodes in an electrochemical cell
b) The difference in the number of electrons in two half-cells
c) The measure of an electrode’s ability to conduct electricity
d) The measure of an electrode’s tendency to lose or gain electrons compared to a standard hydrogen electrode
Answer: d) The measure of an electrode’s tendency to lose or gain electrons compared to a standard hydrogen electrode
8. Faraday’s laws of electrolysis describe the relationship between:
a) Temperature and electrical conductivity
b) Electrode potential and ionic concentration
c) Electric charge and electrode potential
d) Amount of substance produced or consumed during electrolysis and the amount of electric charge passed through the electrolyte
Answer: d) Amount of substance produced or consumed during electrolysis and the amount of electric charge passed through the electrolyte
9. Which law states that the amount of chemical change produced by passing an electric current through an electrolyte is directly proportional to the quantity of electricity passed?
a) Boyle’s law
b) Faraday’s first law of electrolysis
c) Charles’s law
d) Faraday’s second law of electrolysis
Answer: b) Faraday’s first law of electrolysis
10. What is the Nernst equation used for in electrochemistry?
a) To calculate the rate of a chemical reaction
b) To determine the standard electrode potential of a cell
c) To calculate the concentration of ions in a solution
d) To calculate the actual electrode potential under non-standard conditions
Answer: d) To calculate the actual electrode potential under non-standard conditions
11. What is the purpose of cathodic protection in electrochemistry?
a) To prevent oxidation reactions
b) To prevent corrosion of metals
c) To enhance electrode potential
d) To accelerate the rate of chemical reactions
Answer: b) To prevent corrosion of metals
12. Which type of electrochemical cell is used to convert chemical energy directly into electrical energy, with continuous fuel supply?
a) Galvanic cell
b) Electrolytic cell
c) Fuel cell
d) Solar cell
Answer: c) Fuel cell
13. What is the product of the electrolysis of water?
a) Oxygen gas and hydrogen gas
b) Carbon dioxide and hydrogen gas
c) Nitrogen gas and oxygen gas
d) Helium gas and oxygen gas
Answer: a) Oxygen gas and hydrogen gas
14. Which process involves the spontaneous transfer of electrons between substances, resulting in the release of energy?
a) Electroplating
b) Electrolysis
c) Galvanic cell reaction
d) Electrochemical synthesis
Answer: c) Galvanic cell reaction
15. What is the name of the electrode where reduction occurs in an electrochemical cell?
a) Anode
b) Cathode
c) Electromotive force
d) Electrolyte
Answer: b) Cathode
Part 2: Download Electrochemistry questions & answers for free
Download questions & answers for free
16. What is the process called when an external electrical source drives a non-spontaneous redox reaction?
a) Electrolysis
b) Galvanic cell reaction
c) Electrochemical synthesis
d) Electroplating
Answer: a) Electrolysis
17. Which type of electrochemical cell is used in the process of refining copper?
a) Galvanic cell
b) Electrolytic cell
c) Fuel cell
d) Solar cell
Answer: b) Electrolytic cell
18. What happens to the concentration of ions in an electrolyte solution during the process of electrolysis?
a) The concentration increases at the anode and decreases at the cathode.
b) The concentration decreases at the anode and increases at the cathode.
c) The concentration remains constant at both the anode and the cathode.
d) The concentration increases at both the anode and the cathode.
Answer: a) The concentration increases at the anode and decreases at the cathode.
19. Which law states that the mass of a substance produced during electrolysis is directly proportional to the amount of electricity passed through the electrolyte and the molar mass of the substance?
a) Boyle’s law
b) Faraday’s first law of electrolysis
c) Charles’s law
d) Faraday’s second law of electrolysis
Answer: d) Faraday’s second law of electrolysis
20. What is the product of the electrolysis of sodium chloride (NaCl) solution?
a) Hydrogen gas and oxygen gas
b) Sodium metal and chlorine gas
c) Sodium hydroxide and hydrogen gas
d) Chlorine gas and oxygen gas
Answer: b) Sodium metal and chlorine gas
21. What is the process called when a metal surface is protected from corrosion by attaching it to a sacrificial metal that corrodes more readily?
a) Galvanization
b) Anodization
c) Cathodic protection
d) Electrolytic protection
Answer: c) Cathodic protection
22. Which type of electrochemical cell is commonly used to power electronic devices and can be recharged?
a) Galvanic cell
b) Electrolytic cell
c) Fuel cell
d) Rechargeable cell
Answer: d) Rechargeable cell
23. Which of the following is a consequence of oxidation in an electrochemical cell?
a) Reduction of ions at the anode
b) Reduction of ions at the cathode
c) Generation of electrons at the anode
d) Generation of electrons at the cathode
Answer: c) Generation of electrons at the anode
Just to let you know
Sign up for a free OnlineExamMaker account to create an interactive online quiz in minutes – automatic grading & mobile friendly.
24. What is the term for the electrode potential of a cell under standard conditions?
a) Equilibrium potential
b) Galvanic potential
c) Standard potential
d) Reduction potential
Answer: c) Standard potential
25. What is the process called when electrical energy is used to drive a non-spontaneous redox reaction?
a) Electrolysis
b) Galvanic cell reaction
c) Electrochemical synthesis
d) Electroplating
Answer: a) Electrolysis
26. In which type of cell does the oxidation reaction occur at the anode?
a) Galvanic cell
b) Electrolytic cell
c) Fuel cell
d) Solar cell
Answer: a) Galvanic cell
27. What is the unit used to measure the quantity of electricity passed through an electrolyte?
a) Coulomb
b) Volt
c) Ampere
d) Ohm
Answer: a) Coulomb
28. Which type of electrochemical cell is commonly used to convert solar energy into electrical energy?
a) Galvanic cell
b) Electrolytic cell
c) Fuel cell
d) Solar cell
Answer: d) Solar cell
29. What is the name of the process where a metal surface is coated with a thin layer of oxide to improve its resistance to corrosion?
a) Galvanization
b) Anodization
c) Cathodic protection
d) Electrolytic protection
Answer: b) Anodization
30. Which law states that the mass of an element liberated during electrolysis is directly proportional to the quantity of electricity passed and the equivalent weight of the element?
a) Boyle’s law
b) Faraday’s first law of electrolysis
c) Charles’s law
d) Faraday’s second law of electrolysis
Answer: b) Faraday’s first law of electrolysis
Part 3: Free online quiz maker – OnlineExamMaker
Save time and effort with automated grading, instantly generating scores and results for each respondent. OnlineExamMaker grades your quizzes automatically, and provides valuable insights for performance evaluation. Whether you are an educator looking to engage students or a business professional seeking effective training methods, OnlineExamMaker quiz maker is a reliable and efficient solution that streamlines the assessment process for improved learning outcomes.
Create Your Next Quiz/Exam with OnlineExamMaker