Quantum physics is a fundamental branch of physics that describes the behavior of matter and energy at the smallest scales, such as atoms and subatomic particles. It emerged in the early 20th century to explain phenomena that classical physics could not, revolutionizing our understanding of the universe.
Key principles include:
Wave-Particle Duality: Particles like electrons can exhibit both wave-like and particle-like properties, as demonstrated by experiments such as the double-slit experiment.
Superposition: A quantum system can exist in multiple states simultaneously until measured. For example, an electron can be in a superposition of spin-up and spin-down states.
Entanglement: Particles can become linked so that the state of one instantly influences the state of another, regardless of distance, a phenomenon Einstein called “spooky action at a distance.”
Uncertainty Principle: Proposed by Werner Heisenberg, it states that certain pairs of properties, like position and momentum, cannot both be precisely known at the same time.
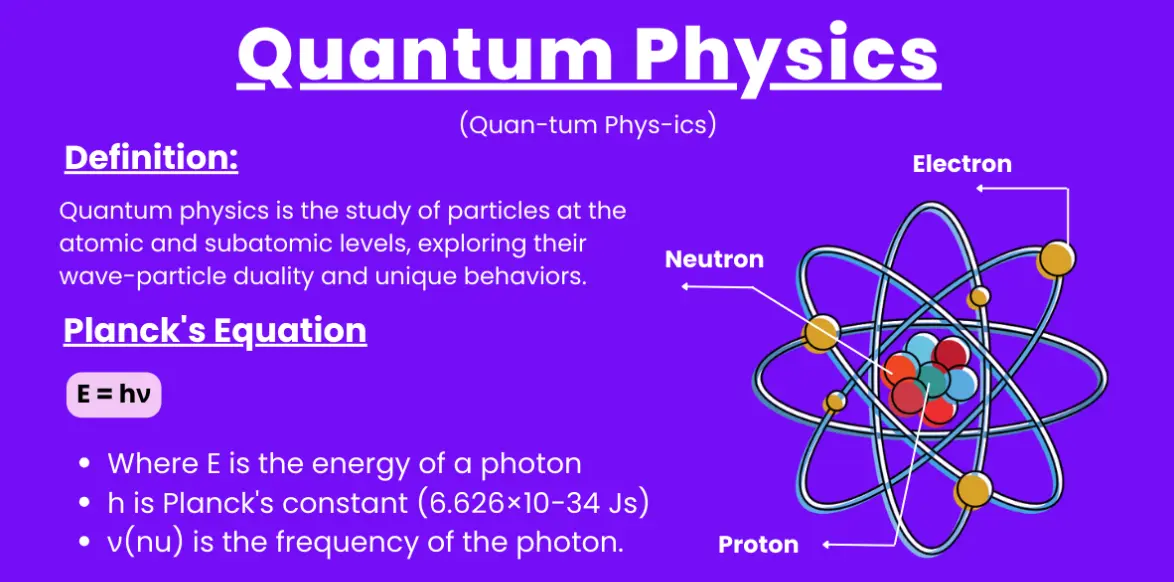
The foundations were laid by pioneers such as Max Planck, who introduced the concept of quantized energy in 1900; Albert Einstein, who explained the photoelectric effect in 1905; Niels Bohr, who developed the atomic model; and Erwin Schrödinger and Heisenberg, who formulated wave mechanics and matrix mechanics in the 1920s.
Table of contents
- Part 1: OnlineExamMaker AI quiz maker – Make a free quiz in minutes
- Part 2: 20 quantum physics quiz questions & answers
- Part 3: Automatically generate quiz questions using AI Question Generator
Part 1: OnlineExamMaker AI quiz maker – Make a free quiz in minutes
Still spend a lot of time in editing questions for your next quantum physics assessment? OnlineExamMaker is an AI quiz maker that leverages artificial intelligence to help users create quizzes, tests, and assessments quickly and efficiently. You can start by inputting a topic or specific details into the OnlineExamMaker AI Question Generator, and the AI will generate a set of questions almost instantly. It also offers the option to include answer explanations, which can be short or detailed, helping learners understand their mistakes.
What you may like:
● Automatic grading and insightful reports. Real-time results and interactive feedback for quiz-takers.
● The exams are automatically graded with the results instantly, so that teachers can save time and effort in grading.
● LockDown Browser to restrict browser activity during quizzes to prevent students searching answers on search engines or other software.
● Create certificates with personalized company logo, certificate title, description, date, candidate’s name, marks and signature.
Automatically generate questions using AI
Part 2: 20 quantum physics quiz questions & answers
or
Question 1:
What is the Heisenberg Uncertainty Principle?
A) It states that the position and momentum of a particle can be known exactly at the same time.
B) It limits the precision with which certain pairs of physical properties, like position and momentum, can be simultaneously known.
C) It describes the wave-like behavior of particles.
D) It explains the quantization of energy levels in atoms.
Answer: B
Explanation: The Heisenberg Uncertainty Principle states that there is a fundamental limit to the accuracy with which we can measure both the position and momentum of a particle simultaneously, arising from the wave nature of particles in quantum mechanics.
Question 2:
Which experiment demonstrates the wave-particle duality of electrons?
A) The photoelectric effect
B) The double-slit experiment
C) Blackbody radiation
D) The Compton effect
Answer: B
Explanation: The double-slit experiment shows that electrons can exhibit interference patterns, indicating wave-like behavior, while also behaving as particles when detected, thus demonstrating wave-particle duality.
Question 3:
What is the ground state of an electron in a hydrogen atom?
A) n = 1
B) n = 2
C) n = 3
D) n = 4
Answer: A
Explanation: In the hydrogen atom, the ground state corresponds to the lowest energy level where the principal quantum number n = 1, representing the most stable state of the electron.
Question 4:
In quantum mechanics, what does the term “superposition” refer to?
A) A particle existing in multiple states simultaneously until measured
B) The entanglement of two particles
C) The collapse of a wave function
D) The quantization of energy
Answer: A
Explanation: Superposition allows a quantum system to exist in a combination of multiple states at the same time, such as an electron being in a mix of spin-up and spin-down states until observed.
Question 5:
What is the de Broglie wavelength associated with a particle?
A) The wavelength of light emitted by the particle
B) The wavelength related to the particle’s momentum
C) The distance between energy levels
D) The radius of the particle’s orbit
Answer: B
Explanation: The de Broglie wavelength is given by λ = h/p, where h is Planck’s constant and p is the particle’s momentum, showing that particles have wave-like properties.
Question 6:
Which principle states that no two electrons in an atom can have the same set of quantum numbers?
A) Heisenberg Uncertainty Principle
B) Pauli Exclusion Principle
C) Aufbau Principle
D) Bohr’s Correspondence Principle
Answer: B
Explanation: The Pauli Exclusion Principle ensures that electrons in an atom occupy different quantum states, which is essential for explaining the structure of the periodic table.
Question 7:
What does the photoelectric effect demonstrate?
A) The wave nature of light
B) The particle nature of light
C) The uncertainty in particle positions
D) Quantum entanglement
Answer: B
Explanation: The photoelectric effect shows that light consists of photons (particles) that can eject electrons from a material, supporting the quantum theory of light as discrete packets of energy.
Question 8:
In quantum entanglement, what happens to two entangled particles?
A) Their states become independent
B) The state of one instantly influences the state of the other, regardless of distance
C) They share the same energy level
D) They exhibit wave-particle duality together
Answer: B
Explanation: Entangled particles are correlated such that measuring the state of one particle immediately determines the state of the other, even if they are separated by large distances, challenging classical notions of locality.
Question 9:
What is the role of the Schrödinger equation in quantum mechanics?
A) It describes the probability of finding a particle in a certain state
B) It calculates the exact path of a particle
C) It explains the uncertainty principle
D) It predicts classical behavior
Answer: A
Explanation: The Schrödinger equation is a wave equation that describes how the quantum state of a system evolves over time, providing the probability distribution for a particle’s position or other properties.
Question 10:
Which quantum number determines the shape of an orbital?
A) Principal quantum number (n)
B) Azimuthal quantum number (l)
C) Magnetic quantum number (m_l)
D) Spin quantum number (m_s)
Answer: B
Explanation: The azimuthal quantum number (l) defines the subshell and thus the shape of the orbital, such as s (spherical) or p (dumbbell-shaped).
Question 11:
What is quantum tunneling?
A) The ability of a particle to pass through a potential barrier it classically shouldn’t
B) The interference of waves in a double-slit setup
C) The collapse of a wave function upon measurement
D) The emission of photons from an atom
Answer: A
Explanation: Quantum tunneling occurs when a particle crosses a potential energy barrier that it does not have enough energy to overcome classically, due to the probabilistic nature of quantum waves.
Question 12:
In the Compton effect, what happens to X-rays scattered by electrons?
A) Their wavelength decreases
B) Their wavelength increases
C) Their frequency remains the same
D) They are absorbed completely
Answer: B
Explanation: The Compton effect demonstrates that X-rays lose energy and increase in wavelength when scattered by electrons, treating photons as particles with momentum.
Question 13:
What is the spin of an electron?
A) 0
B) 1/2
C) 1
D) 3/2
Answer: B
Explanation: Electrons have an intrinsic spin of 1/2, a quantum property that classifies them as fermions and influences their behavior in magnetic fields and atomic structures.
Question 14:
What does the term “wave function” represent in quantum mechanics?
A) The exact position of a particle
B) A mathematical description of the quantum state of a system
C) The energy level of an electron
D) The momentum of a photon
Answer: B
Explanation: The wave function, denoted by ψ, encodes the probability amplitude for finding a particle in various states, and its square gives the probability density.
Question 15:
Which phenomenon is explained by Planck’s constant?
A) The quantization of energy
B) The speed of light
C) The mass of particles
D) The gravitational constant
Answer: A
Explanation: Planck’s constant (h) is fundamental in quantum theory, as it relates the energy of a photon to its frequency (E = hν), leading to the concept of quantized energy levels.
Question 16:
What is the outcome of measuring a quantum system?
A) It creates a superposition
B) It collapses the wave function to a definite state
C) It entangles the system with another
D) It increases the uncertainty
Answer: B
Explanation: Measurement in quantum mechanics causes the wave function to collapse from a superposition of states to a single eigenstate, determining the observed property.
Question 17:
In blackbody radiation, what did Planck propose?
A) Energy is emitted in continuous waves
B) Energy is quantized in discrete packets called quanta
C) Light behaves only as a wave
D) Particles have no wave properties
Answer: B
Explanation: Planck proposed that oscillators in a blackbody emit energy in discrete amounts (quanta), resolving the ultraviolet catastrophe and founding quantum theory.
Question 18:
What is the significance of the Dirac equation?
A) It describes the behavior of photons
B) It combines quantum mechanics with special relativity for electrons
C) It explains the uncertainty principle
D) It predicts the existence of quarks
Answer: B
Explanation: The Dirac equation provides a relativistic description of spin-1/2 particles like electrons, predicting phenomena such as electron spin and antimatter.
Question 19:
How does the uncertainty principle affect everyday objects?
A) It has no effect on macroscopic objects
B) It makes everyday objects behave quantum mechanically
C) It limits the precision of measurements for large objects as well
D) It only applies to subatomic particles
Answer: A
Explanation: For macroscopic objects, the uncertainty is negligible due to their large mass, so quantum effects like the uncertainty principle are not observable in daily life.
Question 20:
What is a qubit in quantum computing?
A) A classical bit that can be 0 or 1
B) A quantum bit that can exist in superposition of 0 and 1 states
C) A unit of energy in quantum systems
D) A measure of entanglement
Answer: B
Explanation: A qubit can represent a superposition of states, allowing quantum computers to process information in parallel, unlike classical bits.
or
Part 3: Automatically generate quiz questions using OnlineExamMaker AI Question Generator
Automatically generate questions using AI