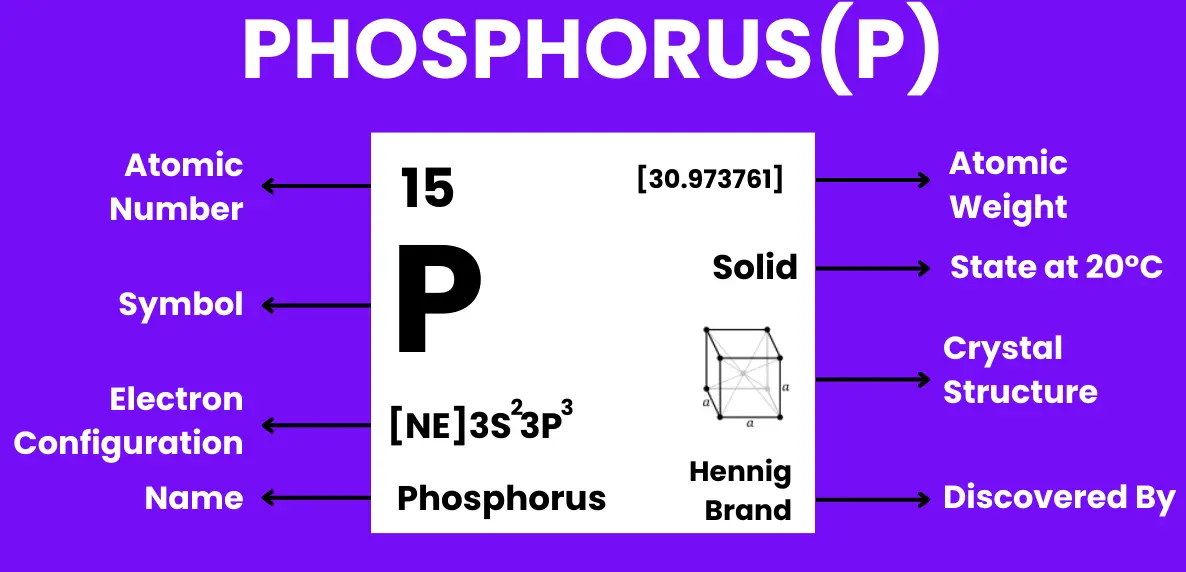
Phosphorus is a chemical element with the symbol P and atomic number 15. It is a non-metal in the nitrogen group of the periodic table, essential for life as it forms a key component of biological molecules such as DNA, RNA, ATP (adenosine triphosphate), and phospholipids in cell membranes.
Discovered in 1669 by German alchemist Hennig Brand through the distillation of urine, phosphorus is the 11th most common element on Earth and does not occur naturally in its pure form due to its high reactivity. It exists in several allotropes, including:
– White phosphorus: A waxy, translucent solid that is highly toxic and ignites spontaneously in air. It is used in military applications, such as smoke bombs, and in the production of matches and fertilizers.
– Red phosphorus: More stable and less reactive than white phosphorus, it is commonly used in safety matches, fireworks, and as a flame retardant.
– Black phosphorus: A layered material with a graphite-like structure, known for its electrical conductivity and potential applications in electronics, such as transistors.
Phosphorus is crucial for agriculture, primarily as a key ingredient in fertilizers like superphosphate, which enhances soil fertility and promotes plant growth. It is also vital in industrial processes, including the manufacture of phosphoric acid for detergents, food additives, and water treatment chemicals.
In the human body, phosphorus supports bone and teeth formation, energy transfer, and cellular functions. However, excessive intake or environmental pollution from phosphorus runoff can lead to issues like eutrophication in water bodies.
Globally, phosphorus reserves are finite, primarily extracted from phosphate rock, raising concerns about sustainability and the need for recycling and efficient use in various sectors.
Table of Contents
- Part 1: OnlineExamMaker AI Quiz Maker – Make A Free Quiz in Minutes
- Part 2: 20 Phosphorus Quiz Questions & Answers
- Part 3: Automatically Generate Quiz Questions Using AI Question Generator
Part 1: OnlineExamMaker AI Quiz Maker – Make A Free Quiz in Minutes
What’s the best way to create a Phosphorus quiz online? OnlineExamMaker is the best AI quiz making software for you. No coding, and no design skills required. If you don’t have the time to create your online quiz from scratch, you are able to use OnlineExamMaker AI Question Generator to create question automatically, then add them into your online assessment. What is more, the platform leverages AI proctoring and AI grading features to streamline the process while ensuring exam integrity.
Key features of OnlineExamMaker:
● Create up to 10 question types, including multiple-choice, true/false, fill-in-the-blank, matching, short answer, and essay questions.
● Build and store questions in a centralized portal, tagged by categories and keywords for easy reuse and organization.
● Automatically scores multiple-choice, true/false, and even open-ended/audio responses using AI, reducing manual work.
● Create certificates with personalized company logo, certificate title, description, date, candidate’s name, marks and signature.
Automatically generate questions using AI
Part 2: 20 Phosphorus Quiz Questions & Answers
or
1. Question: What is the atomic number of phosphorus?
A) 13
B) 14
C) 15
D) 16
Answer: C) 15
Explanation: Phosphorus has an atomic number of 15, indicating it has 15 protons in its nucleus.
2. Question: What is the chemical symbol for phosphorus?
A) P
B) Ph
C) Po
D) Ps
Answer: A) P
Explanation: The chemical symbol for phosphorus is P, as designated by the International Union of Pure and Applied Chemistry (IUPAC).
3. Question: Who is credited with discovering phosphorus?
A) Antoine Lavoisier
B) Hennig Brand
C) Marie Curie
D) Dmitri Mendeleev
Answer: B) Hennig Brand
Explanation: Hennig Brand isolated phosphorus in 1669 from urine while searching for the Philosopher’s Stone.
4. Question: Which allotrope of phosphorus is highly reactive and flammable?
A) Black phosphorus
B) Red phosphorus
C) White phosphorus
D) Violet phosphorus
Answer: C) White phosphorus
Explanation: White phosphorus is the most reactive form, igniting spontaneously in air due to its tetrahedral structure.
5. Question: In which group of the periodic table is phosphorus located?
A) Group 14
B) Group 15
C) Group 16
D) Group 17
Answer: B) Group 15
Explanation: Phosphorus is in Group 15 (p-block), also known as the nitrogen group, due to its five valence electrons.
6. Question: What is the most common oxidation state of phosphorus in compounds?
A) +1
B) +3
C) +5
D) -3
Answer: C) +5
Explanation: Phosphorus commonly exhibits a +5 oxidation state in compounds like phosphates, forming stable bonds with oxygen.
7. Question: Phosphorus is essential for which biological molecule?
A) Proteins
B) Lipids
C) DNA
D) Carbohydrates
Answer: C) DNA
Explanation: Phosphorus is a key component of DNA’s phosphate backbone, helping to form the sugar-phosphate structure.
8. Question: Which form of phosphorus is used in safety matches?
A) White phosphorus
B) Red phosphorus
C) Black phosphorus
D) Yellow phosphorus
Answer: B) Red phosphorus
Explanation: Red phosphorus is used on the striking surface of safety matches because it is less reactive than white phosphorus.
9. Question: What is the electron configuration of a phosphorus atom?
A) 1s² 2s² 2p⁶ 3s² 3p³
B) 1s² 2s² 2p⁶ 3s² 3p⁴
C) 1s² 2s² 2p⁶ 3s¹ 3p⁵
D) 1s² 2s² 2p⁶ 3s² 3p²
Answer: A) 1s² 2s² 2p⁶ 3s² 3p³
Explanation: Phosphorus has 15 electrons, with the configuration ending in 3s² 3p³, reflecting its position in the periodic table.
10. Question: Phosphorus is primarily obtained from which mineral?
A) Halite
B) Gypsum
C) Apatite
D) Quartz
Answer: C) Apatite
Explanation: Apatite, a phosphate mineral, is the main source of phosphorus for fertilizers and industrial uses.
11. Question: Which compound is a major component of fertilizers?
A) Sodium chloride
B) Calcium phosphate
C) Carbon dioxide
D) Sulfur dioxide
Answer: B) Calcium phosphate
Explanation: Calcium phosphate provides phosphorus for plant growth and is widely used in agricultural fertilizers.
12. Question: What is the physical state of white phosphorus at room temperature?
A) Gas
B) Liquid
C) Solid
D) Plasma
Answer: C) Solid
Explanation: White phosphorus is a waxy solid at room temperature but is highly toxic and must be stored under water.
13. Question: Phosphorus plays a key role in which energy-carrying molecule?
A) Glucose
B) ATP
C) Hemoglobin
D) Insulin
Answer: B) ATP
Explanation: Adenosine triphosphate (ATP) contains phosphorus in its phosphate groups, which store and transfer energy in cells.
14. Question: Which isotope of phosphorus is commonly used in radioactive tracing?
A) Phosphorus-30
B) Phosphorus-31
C) Phosphorus-32
D) Phosphorus-33
Answer: C) Phosphorus-32
Explanation: Phosphorus-32 is a beta-emitter used in biological research for labeling DNA and studying metabolic processes.
15. Question: What happens when phosphorus reacts with oxygen?
A) It forms carbon dioxide
B) It forms phosphorus oxides
C) It forms water
D) It forms nitrogen gas
Answer: B) It forms phosphorus oxides
Explanation: Phosphorus reacts with oxygen to produce oxides like P4O6 and P4O10, depending on the conditions.
16. Question: Phosphorus deficiency in plants can lead to what symptom?
A) Yellowing of leaves
B) Stunted growth
C) Excessive flowering
D) Increased fruit production
Answer: B) Stunted growth
Explanation: Phosphorus is vital for energy transfer and root development, so deficiency often results in poor growth and dark green leaves.
17. Question: Which environmental issue is associated with excess phosphorus?
A) Acid rain
B) Eutrophication
C) Ozone depletion
D) Global warming
Answer: B) Eutrophication
Explanation: Excess phosphorus from runoff causes algal blooms in water bodies, leading to oxygen depletion and ecosystem damage.
18. Question: What is the melting point of red phosphorus?
A) 44°C
B) 280°C
C) 590°C
D) 800°C
Answer: C) 590°C
Explanation: Red phosphorus has a higher melting point of about 590°C compared to white phosphorus, making it more stable.
19. Question: Phosphorus is used in the production of which material?
A) Plastics
B) Matches and fireworks
C) Glass
D) Paper
Answer: B) Matches and fireworks
Explanation: Phosphorus compounds are used in matches for ignition and in fireworks for their flame-retardant properties.
20. Question: How many valence electrons does phosphorus have?
A) 3
B) 4
C) 5
D) 6
Answer: C) 5
Explanation: Phosphorus, in Group 15, has 5 valence electrons in its outer shell, allowing it to form three bonds and have a lone pair.
or
Part 3: Automatically generate quiz questions using OnlineExamMaker AI Question Generator
Automatically generate questions using AI